Products
Lomotral - Oral Powder
Therapeutic Category : Anti-diarrhoeal + Oral Rehydration Salts
Therapeutic Category : Anti-diarrhoeal + Oral Rehydration Salts
Actions
Lomotral controls diarrhoea with synergistic actions of botanicals Kutaja (Holarrhena antidysenterica), Vidanga (Embelia ribes)
and Daruhalad (Berberis aristata) by:
- Enhancing intestinal capillary micro-circulation to stimulate water absorption,
- Reducing electrolyte secretion,
- Mildly reducing gut motility,
- Increase colonic water & electrolyte re-absorption and
- Inactivating some of the diarrhoea inducing microbial toxins.
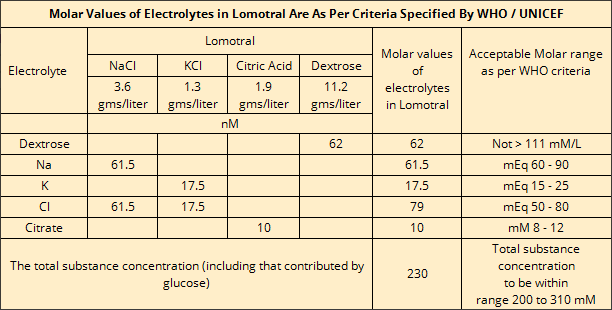
Lomotrol also replenishes vital electrolytes including sodium, potassium, citrates & glucose. The molar values of electrolytes
in Lomotral are in keeping with the WHO / UNICEF defined criteria of acceptable molar range for electrolytes in ORS formulations.
Indications
In infants, children and adults :
- Diarrhoeas of varied etiologies (Atisara, Balatisara)
- Dysentery (Pravahika)
- Dehydration (Nirjalana)




