Quality Assurance
Quality assurance imbibes confirmation and validation of identity, purity, content, and other chemical, physical, or
biological properties, and is therefore of paramount importance in ensuring batch-to-batch uniformity, efficacy and safety
of finished products.
We are committed to deliver quality products and have integrated our systems to the doctrine of “Quality Assurance” over “Quality Control”. This entails “Quality” to be built-in as an intrinsic attribute right from the cultivation, harvesting & collection stage through the raw material inward stage, through production processes unto the finished product stage, through validated checks & procedures assuring product quality through its entire shelf-life.
We adhere to “Quality Assurance Parameters” for herbal and traditional therapies including ayurveda, siddha and unani drugs evolved by the WHO and other academic centers of excellence across the globe.
A battery of tests encompassing the domains of chemistry, botany, pharmocognosy & sophisticated methods of instrumental analysis all contribute in quality assurance of herbal & natural remedies.
Thus the methods for quality assurance of herbal & natural remedies involve sensory inspection (macroscopic and microscopic examinations) and analytical inspection using instrumental techniques such as thin layer chromatography, HPLC, GC–MS, LC–MS, near infrared (NIR), and spectrophotometer.




Botanical Tests
A single herbal drug may contain a great many natural chemical constituents, and traditional & natural herbal remedies normally contain a combination of herbal drugs, and therefore an even larger number of natural chemical constituents. Further a combination of several herbs during manufacturing processes might give rise to de novo phytochemical moieties. Therefore the finished herbal formulation would have a very profile of active &/or inactive chemical moieties present. It is the underlying philosophy of traditional herbal medicine that these multiple constituents work ‘synergistically’ and in symphony and all contribute to the biological effects of the finished products and could hardly be separated into active parts.
In the past, standardization of herbal medicinal finished products was done by qualitatively / quantitatively assaying one or two active / inactive markers in the finished formulation. This kind of the determination, however, does not give a complete picture of a herbal product, because multiple constituents which are present and may contribute to the end biological effects are not mapped or accounted for.
Moreover, the chemical constituents in component herbs in the compound herbal formulations may vary depending on harvest seasons, plant origins, drying processes and other factors. Thus, it seems to be necessary to determine most of the phytochemical constituents of herbal products, in other words their phytochemical profile, in order to ensure the reliability, batch-to-batch uniformity & reproducibility of their pharmacological effects in the finished formulations.
Thus, several chromatographic techniques, such as high-performance liquid chromatography (HPLC), gas chromatography (GC), capillary electrophoresis (CE) and thin layer chromatography (TLC), can be applied for this kind of documentation. In this way, the full herbal product could be regarded as the active ‘compound’. Accordingly a chemical profile, such as a chromatographic fingerprint presenting a relatively good integral representation of various chemical components for a finished product should be constructed and compared with the profile of a standard reference batch product to ensure batch-to-batch uniformity.
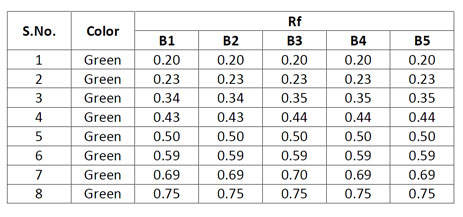
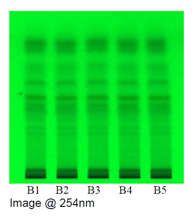
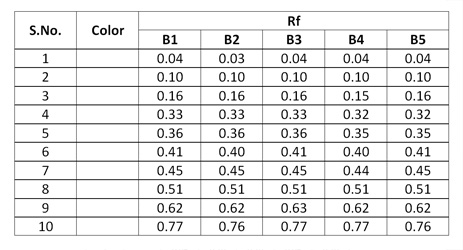
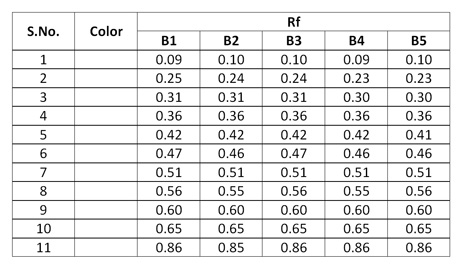
In TLC fingerprinting, the data that can be recorded using a high-performance TLC (HPTLC) scanner includes the chromatogram, retardation factor (Rf) values, the color of the separated bands, their absorption spectra, ?max of all the resolved bands. All of these, together with the profiles on derivatization with different reagents, represent the TLC fingerprint profile of the sample. The fingerprint represents the evidence for identification of an authentic drug, in excluding the adulterants and in maintaining the quality and consistency of the drug.
Quality Assurance TLC Fingerprint of Finished Tablet Formulation: An Example
Method
A predefined aliquot of sample (pre-defined solvent extract of tablets) from each batch of tablets was applied on Merck Aluminum plate pre-coated with silica gel 60 F254 precoated 10 x 10 cm. The plate was developed in Toulene:Chloroform:Ethanol (4:4:1) (v/v/v). The plate was dried and visualized in UV 254. The plate was dipped in Anisaldehyde Sulphuric acid reagent and heated till spots appeared and visualized in visible light & in UV 366 nm.
Observations under UV 254 nm
Observations after derivatization under visible light
Observations after derivatization under UV 366 nm
We are committed to deliver quality products and have integrated our systems to the doctrine of “Quality Assurance” over “Quality Control”. This entails “Quality” to be built-in as an intrinsic attribute right from the cultivation, harvesting & collection stage through the raw material inward stage, through production processes unto the finished product stage, through validated checks & procedures assuring product quality through its entire shelf-life.
We adhere to “Quality Assurance Parameters” for herbal and traditional therapies including ayurveda, siddha and unani drugs evolved by the WHO and other academic centers of excellence across the globe.
A battery of tests encompassing the domains of chemistry, botany, pharmocognosy & sophisticated methods of instrumental analysis all contribute in quality assurance of herbal & natural remedies.
Thus the methods for quality assurance of herbal & natural remedies involve sensory inspection (macroscopic and microscopic examinations) and analytical inspection using instrumental techniques such as thin layer chromatography, HPLC, GC–MS, LC–MS, near infrared (NIR), and spectrophotometer.




Botanical Tests
- Botanical description
- Geographical area/s & season/s of collection
- Climatic & soil requirements
Pharmacognostic Tests
- Organoleptic (Colour, Odour, Taste, Fracture/ texture)
- Macroscopy
- Microscopy
- Phytochemical groupings tests
- Phenols
( Simple phenols, Phenylpropanoids, Salicylates and salicins, Lignans, Coumarins, Stilbenes, Quinones, Miscellaneous phenolic compounds ) - Polyphenols—tannins and flavonoids
( Tannins, Flavonoids, Anthocyanins ) - Glycosides
( Cyanogenic glycosides, Phenylpropanoid glycosides 4, Anthraquinones, Glucosinolates, Iridoid glycosides) - Terpenes
( Monoterpenes, Sesquiterpenes, Diterpenes, Bitter principles, Triterpenes, Tetraterpenes) - Triterpenoids and saponins
( Phytosterols, Saponins, Cardiac glycosides, Free triterpenes) - Essential oils and resins
( Essential oils, Resins) - Fixed oils and alkamides
( Omega 3 and 6 essential fatty acids, Alkamides) - Polysaccharides
( Gums, Pectins, Mucilages, Fructans) - Alkaloids
( Pyridine-piperidine alkaloids, Quinoline alkaloids, Isoquinoline alkaloids, Tropane alkaloids, Quinolizidine alkaloids, Pyrrolizidine alkaloids, Indole alkaloids, Steroidal alkaloids, Alkaloidal amines, Purine alkaloids)
Physico-chemical Tests
- Loss on drying at 105o C
- Total- ash
- Acid insoluble ash
- Alcohol soluble extractive
- Water soluble extractive
- pH
- Qualitative / quantitative assay for active / inactive marker(s)
Instrumental Analytical
Techniques
- TLC/HPTLC fingerprinting
A single herbal drug may contain a great many natural chemical constituents, and traditional & natural herbal remedies normally contain a combination of herbal drugs, and therefore an even larger number of natural chemical constituents. Further a combination of several herbs during manufacturing processes might give rise to de novo phytochemical moieties. Therefore the finished herbal formulation would have a very profile of active &/or inactive chemical moieties present. It is the underlying philosophy of traditional herbal medicine that these multiple constituents work ‘synergistically’ and in symphony and all contribute to the biological effects of the finished products and could hardly be separated into active parts.
In the past, standardization of herbal medicinal finished products was done by qualitatively / quantitatively assaying one or two active / inactive markers in the finished formulation. This kind of the determination, however, does not give a complete picture of a herbal product, because multiple constituents which are present and may contribute to the end biological effects are not mapped or accounted for.
Moreover, the chemical constituents in component herbs in the compound herbal formulations may vary depending on harvest seasons, plant origins, drying processes and other factors. Thus, it seems to be necessary to determine most of the phytochemical constituents of herbal products, in other words their phytochemical profile, in order to ensure the reliability, batch-to-batch uniformity & reproducibility of their pharmacological effects in the finished formulations.
Thus, several chromatographic techniques, such as high-performance liquid chromatography (HPLC), gas chromatography (GC), capillary electrophoresis (CE) and thin layer chromatography (TLC), can be applied for this kind of documentation. In this way, the full herbal product could be regarded as the active ‘compound’. Accordingly a chemical profile, such as a chromatographic fingerprint presenting a relatively good integral representation of various chemical components for a finished product should be constructed and compared with the profile of a standard reference batch product to ensure batch-to-batch uniformity.
In TLC fingerprinting, the data that can be recorded using a high-performance TLC (HPTLC) scanner includes the chromatogram, retardation factor (Rf) values, the color of the separated bands, their absorption spectra, ?max of all the resolved bands. All of these, together with the profiles on derivatization with different reagents, represent the TLC fingerprint profile of the sample. The fingerprint represents the evidence for identification of an authentic drug, in excluding the adulterants and in maintaining the quality and consistency of the drug.
Quality Assurance TLC Fingerprint of Finished Tablet Formulation: An Example
Method
A predefined aliquot of sample (pre-defined solvent extract of tablets) from each batch of tablets was applied on Merck Aluminum plate pre-coated with silica gel 60 F254 precoated 10 x 10 cm. The plate was developed in Toulene:Chloroform:Ethanol (4:4:1) (v/v/v). The plate was dried and visualized in UV 254. The plate was dipped in Anisaldehyde Sulphuric acid reagent and heated till spots appeared and visualized in visible light & in UV 366 nm.
Observations under UV 254 nm
 |
 |
Observations after derivatization under visible light
 |
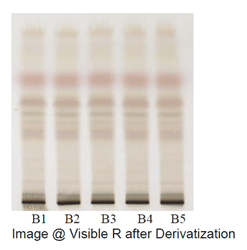 |
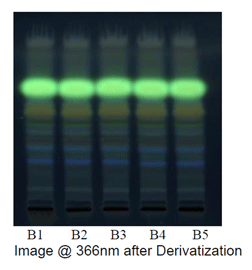
Observations after derivatization under UV 366 nm
 |
|
Negative Markers & Their
Maximum Permissible Limits
In addition to the desirable or positive parameters of standardization enlisted above, a list of negative attributes along with the maximum permissible limits in the finished products (heavy metal, microbial count, pesticide residue, aflatoxins) have also been developed.
In addition to the desirable or positive parameters of standardization enlisted above, a list of negative attributes along with the maximum permissible limits in the finished products (heavy metal, microbial count, pesticide residue, aflatoxins) have also been developed.
- Microbial contamination limits in medicinal plant materials
- For contamination of "crude" plant material harvested under acceptable
hygienic conditions:
( Escherichia coli maximum 104 per gram; mould propagules maximum 105 per gram. ) - For plant materials that have been pretreated (e.g. with boiling water as
used for herbal teas and infusions) or that are used as topical dosage
forms:
( aerobic bacteria maximum 107 per gram; yeasts and moulds maximum 104 per gram; Escherichia coli maximum 102 per gram; other enterobacteria maximum 104per gram; salmonellae none. ) - For other plant materials for internal use :
( Aerobic bacteria maximum 105 per gram; yeasts and moulds maximum 103 per gram; Escherichia coli maximum 10 per gram; other enterobacteria maximum 103per gram; salmonellae none.) - Heavy metals permissible limits
- Lead <10.0 ppm
- Cadmium <0.30 ppm
- Mercury <1.00 ppm
- Arsenic <10.0 ppm
- Pesticides residue limits
- Aflatoxins limits